Miter Gauge Upgrade Failed,2x4 Furniture Plans Free Pdf Pdf,Carvey Cnc Carving Machine Package - Easy Way
26.03.2021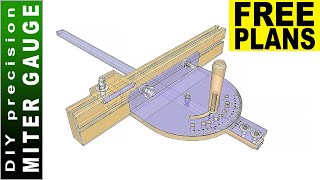
Equipment intended to be chemically sanitized shall be designed to ensure contact between process contact surfaces and the sanitization solution.
The properties of the filter elements shall be considered to confirm compatibility of the element with the exposure conditions of a thermal sanitization process. All process piping systems that include piping, tubing, and fluidic components shall be sloped for adequate drainage. For all low points in the system, a drain port shall be installed.
A common drain port on the skid is preferred. For this section, "system" is intended to cover the chromatography piping skid, not including the associated column. Chromatography systems shall be designed for cleaning in place. Systems should be designed in accordance with SD Chemical sanitization processes are used to reduce bioburden. All process contact surfaces of system components shall either be compatible with the selected sanitization agents or be capable of being removed or isolated prior to the sanitization process.
Chromatography systems are typically stored flooded with a sanitizing solution to maintain bioburden control. Chromatography systems may be designed for thermal sanitization.
However, because it is generally not possible to perform thermal sanitization of columns, the requirement is often waived for chromatography systems. If a system is 78 designed for thermal sanitization, components shall be designed for the specified conditions, or shall be removed or isolated prior to the sanitization process.
Note that if items are removed for sanitization, they should be sanitized separately and reinstalled in a controlled environment to avoid contaminating the system. This section describes the requirements for washers that are designed to clean various materials and components such as glassware, drums, containers, hoses, pallets, and accessories washable items that are not cleaned in place.
Requirements in this section are intended to be applied to cabinet washers, but may be applied to other types of washers as appropriate. Cabinet washers may be designed with an integrated chemical addition system or receive cleaning solutions from a CIP system. If a direct utility connection is used, the design should mitigate the effect of variation in supply pressure e. The break-tank shall be self-drainable and vented. Rinse water from the break-tank shall not contribute to the soiling or bioburden load in the cabinet.
These surfaces, which have the potential to drip onto washed items, shall have complete spray coverage see SD Internal surfaces that may be difficult to clean e. Verification methods and acceptance criteria for drainability shall be agreed on in advance by all the parties. Surface finish verification may not be possible for all components of the loading rack.
A prefilter and HEPA filter system are recommended to protect the washed items. Design of spray systems in cabinet washers requires the integration of manifolded spray devices in the chamber with those installed in loading racks.
Spray systems in cabinet washers may use both static and dynamic spray devices that comply with SD The design considerations should include positive identification of each chemical delivery and connection.
The design of concentrated chemical delivery and storage systems should consider minimizing human contact. These tests apply to newly installed systems and to modifications of existing systems e. Cabinet washers should be tested to confirm complete spray coverage of the specified washable items and the interior process contact surfaces of the washer chamber.
The spray device coverage testing described in SD The spray device coverage test procedure described in Nonmandatory Appendix L may be used for cabinet washers with the following additional considerations: a Testing should include empty configurations i. If parts are dry when inspected, they should be gently rewetted with ambient or cold water to observe any residual riboflavin fluorescence.
The proposed drainability test procedure in SD The chamber should be wetted by liquid delivered through the spray system. The performance test should demonstrate the ability to clean loaded items based on an initial list of washable items agreed to by the end-user and manufacturer. The test should verify removal of residue from surfaces and that the final rinse meets the specified water quality e.
The test should verify that the process contact surfaces within the washer are also cleaned to the same specifications used for the washable items. For this section, "autoclaves" and "steam sterilizers" shall be used synonymously. This section describes the requirements of autoclaves that are used in bioprocessing for the steam sterilization of hard, dry-wrapped, and liquid materials. The chambers shall also be vacuum rated.
This section does not pertain to pasteurizers, ETO ethylene oxide , VHP vaporized hydrogen peroxide , or ClO 2 chlorine dioxide type sterilization equipment. The manufacturer shall define the sterile boundary of the system. Autoclaves should be capable of multiple cycle types for various load conditions. Autoclaves shall only be used to sterilize the types of goods for which they are designed.
The most common load types are specified in SD Effective removal of noncondensable gases is required for effective autoclaving of hard goods. Hard goods may be wrapped or unwrapped. Unwrapped goods can often be effectively autoclaved using either a single vacuum pull or gravity air displacement.
These goods can sometimes be autoclaved at higher temperatures. Multiple vacuum pulse preconditioning is required for wrapped goods to ensure proper evacuation of noncondensable gases from both the autoclave chamber and autoclaved goods. Steam sterilizers used for the processing of wrapped or porous goods shall be able to pull vacuum to levels below 1 psia [69 mbar a ] and maintain the vacuum with a maximum leak rate of 0. Cooling, drying pulse, vacuum is an optional cycle step used to dry goods at the end of the autoclave cycle.
Heated pulse drying is also recommended for the drying of porous goods such as rubber stoppers. Exhaust rates and heating rates should be adjustable for pressuresensitive materials. Forced air removal preconditioning is an optional cycle used to evacuate 82 the noncondensable gases from the autoclave chamber. Liquid cooling cycles should be provided to efficiently cool the autoclave chamber.
Providing the chamber with overpressure helps prevent the liquid goods from boiling over during the cool-down phase. Liquids can also be cooled by slow-rate exhaust. Heating rates should be adjustable to help compensate for differences in heating profiles of items in mixed loads. An independent air filter SIP sterilization cycle should be provided for the in situ sterilization of the chamber vent filters ensuring supply of sterile air for cool-down phases of autoclave loads.
Materials in contact withsteam shall resist corrosion from steam and steam condensate. The materials shall not affect steam quality and shall not release any substances known to be toxic or that could adulterate the product. Tubing within the sterile boundary should be orbital-welded stainless steel tubing where possible and shall comply with Part MJ Table MJ All process contact surfaces within the sterile boundary including tubing, chamber, and components shall be passivated.
The autoclave shall be enclosed with paneling that is resistant to corrosion and is cleanable. The surface finish within the sterile boundary need not exceed 35 R a in. Electropolishing is not required for steam sterilization systems. Elastomers shall comply with SG Elastomers shall be resistant to corrosion and to chemical and thermal degradation. Seals should meet the testing requirements specified in SG External surfaces should be insulated to minimize heat transmission.
Autoclave door s shall be accessible, cleanable, and replaceable, and should be capable of undergoing inspection without dismantling. The door seal shall be resistant to clean steam and clean steam condensate.
The door on the nonsterile side shall be capable of reopening after closing without undergoing a cycle. The door s shall not be capable of opening during a sterilization cycle. The doors shall be constructed of materials that are resistant to clean steam and clean steam condensate.
For multiple-door systems, the doors shall be interlocked to allow the opening of only one door at a time. The unloading "sterile-side" door shall remain sealed in standby mode. Refer to Part SG for specifications of seals used in bioprocessing. Where the sterilization cycle requires admission of air into the chamber, the air should be filtered with a sterilizing filter 0. The filter element shall be replaceable. Provisions for the steam in place of the vent filter elements should be provided.
Refer to SD Carts and trays exposed to clean steam shall be constructed of materials resistant to clean steam and clean steam condensate. Carts, trays, and chamber shall be accessible or removable and cleanable. Valves and sealing materials located within the sterile boundary shall comply with SG Valves within the sterile boundary are typically only exposed to clean steam service and chemical s used during passivation.
Exposure to these conditions should be considered when selecting a valve type for this application. Provisions to prevent back-siphoning into the service feed systems should be considered. The jacket shall be constructed using materials that are resistant to corrosion and degradation from steam or clean steam and clean steam condensate, as applicable.
Autoclave pressure and temperature shall be displayed at all doors. All instruments within the sterile boundary should be of hygienic design. Instruments shall be capable of being calibrated and replaced. The instrumentation shall include the following: a Temperature. Independent temperature elements one or two for monitoring and recording and an independent one for controlling temperature shall be provided.
The chamber temperature recording element should be located in the chamber drain. The element installation shall not affect the maximum leak rate. The temperature elements shall be temperature and clean steam resistant. The pressure instruments shall monitor the chamber and jacket pressures.
Provisions for 83 recording chamber pressure during active autoclave cycles shall be included. Provisions for recording the date and time during an autoclave cycle shall be included.
Multiple paths within a circuit may be cleaned simultaneously. The CIP skid should be designed to deliver feed water, inject cleaning chemicals, heat, and supply the cleaning solution to the soiled equipment. The skid shall also be designed to remove all residual cleaning chemicals added during the cycle. The distribution equipment may also return the solution to the CIP skid, if applicable. Provision for separation of feed waters and wash solutions should be considered.
CIP skids may be located in a fixed, centralized location or may be portable and used adjacent to the soiled equipment. The tank s shall be designed for cleanability per SD Chemical segregation, spill control, addition handling, material compatibility, secondary containment, and personnel safety should be considered. The CIP flow rate should be greater than the process flow rate. The flow direction, line orientation, line size, and presence and orientation of branches, fittings, and other equipment can have a significant influence on the flow rate required to remove air.
NOTE: Factors that may mitigate the risk of insufficient cleaning due to incomplete air removal from branches include a CIP flow rates higher than process flow rates are likely to wet all surfaces that were soiled. Spray devices shall be designed and fabricated per SD Some appurtenances may require additional provisions for cleaning.
If not removed during CIP, cleaning solutions shall flow through appurtenances to clean their interior. Proper hydraulic balance supply and return flow of the CIP circuit and sizing of the bottom outlet valve should be considered to minimize fluid level.
The installation of a vortex breaker may be required. Vortex breaker surfaces shall be sloped to eliminate pooling during CIP and positioned to not adversely affect the hydraulic balance of the CIP circuit. Slope designation GSD2 is recommended. The entire ring path is cleaned during a CIP cycle. Transfer panels shall be designed and fabricated per SD For this section, a CIP distribution "multiport valve" shall be defined as a multiple valve assembly fabricated as a single body to minimize distances and maximize drainability [see.
Centrifugal pumps are preferred for CIP return applications. For this section, a CIP "return eductor" shall be defined as a device that uses a motive fluid to create a pressure differential that returns the CIP solution.
The thermal treatment systems described here reduce or eliminate viable microorganisms and viruses in a liquid under continuous flow conditions. Treatment of process liquids using high temperature for a short residence time may be desired to minimize degradation of the product or a product intermediate.
Thermal systems may be designed to achieve goals that do not require sterilization of the process liquid, such as inactivation of viruses or a particular bacterial species. Bio-inactivation waste treatment systems and food pasteurization systems are not included in the scope of this section. Treatment conditions i. Direct steam injection is performed with a steam injector valve typical or in an infusion chamber. These elements heat the process liquid to the required temperature. Heat exchangers shall be designed to meet the performance requirements in SD Energy recovery heat exchangers use either direct or indirect recovery of energy residing in heated process liquid flow by preheating incoming process liquids with 16 exiting treated liquid.
Direct recovery uses one heat exchanger for this purpose, typically tube-in-tube, which shall be hygienic on both hot and cold sides. Indirect recovery uses two exchangers with a recirculated heat transfer fluid to prevent process liquid contamination.
Heat exchangers in HTST and UHT applications are typically more challenging to clean than heat exchangers in most other bioprocess applications. The following types of heat exchangers may be used in thermal treatment systems: a Shell and Tube. Shell-and-tube heat exchangers may be straight tube or U-tube. The effect of bypass through the bonnet drain slots and slippage between the bonnet and tube sheet shall be considered in thermal design of the heat exchanger.
Coil heat exchangers shall be installed in a self-drainable vertical orientation. Electric heat exchangers shall be designed to provide uniform heating, for example, where electric current is applied directly to the process-contacting tube. Process liquid, typically in the inner tube, is enclosed in an outer tube containing counterflow heating or cooling fluid.
See cautions in SD A sight glass installed downstream of the steam injector is recommended to confirm single-phase flow. Where the steam injection system is designed for CIP, it shall be drainable and exposed to CIP solution across the seat of the steam injection valve.
As an example, Fig. In the figure, to ensure that less than 1 particle out of 10 12 has less than the sec required residence time at 9. Actual retention tube geometry, such as the number and radii of elbows, coils, or U-bends, may impact the results.
The flow rate shall be controlled and monitored to ensure proper system operation. The system shall be designed to ensure that pressure downstream of the heating exchanger or steam injector is above the ASME BPE 1 "Not fully treated" fluid is defined as fluid whose retention time is less than the required retention time.
A pressure of at least 10 psi 0. Thermal treatment systems shall be capable of the following functions. The process contact portions of the system shall be drainable per SD Thermal treatment systems shall be designed for cleaning in place of process contact surfaces. Different elements within the system may have different cleaning requirements or procedures. Where fouling of heated surfaces may occur, cleaning and operational procedures should address potential fouling of those segments of the system.
When compendial water from a break-tank is used in nonrecirculating mode to condition and flush the system, provision shall be made for the sanitization of the water tank and its piping at a minimum. If sterilization of the thermal treatment system is required, the area within 92 the sterile envelope or boundary shall be designed accordingly.
Sterilization may be provided by conditioning the system with liquid at exposure temperature for the required duration, by chemical sterilization, or with a SIP cycle. The initial liquid passing through the thermal treatment system is not expected to meet the required treatment parameters. The system shall be capable of a priming operation to fill the system with liquid, remove air, and establish pressure and flow control.
The thermal treatment system may be primed by the process liquid to be treated or by a priming liquid e. UHT systems shall be sanitized. HTST systems should be sanitized when needed to ensure that the treated process liquid is not compromised.
An HTST system may not require sanitization in all cases. For example, if the system is cleaned and primed using WFI to achieve treatment temperatures, the WFI used for startup may not pose a risk of contamination of the downstream piping. Thermal treatment systems shall be designed to stabilize the temperature of the liquid before diverting it to the destination.
Heating, cooling, flow rate, and back pressure controls are enabled and allowed to stabilize. If the system uses a priming liquid, such as WFI, the system shall stabilize the temperature using the priming liquid and then transition to and restabilize using the process liquid. Stabilization using the process liquid should continue until all the priming liquid has been cleared from the system, at which point the system shall divert the heat-treated process liquid to the destination.
The system shall deliver heat-treated process liquid to the destination only if the performance requirements are met. If they are not met, the system shall divert the liquid to another destination typically to a drain or a collection vessel.
The heat treatment continues until the desired amount of liquid is treated. At the conclusion of the process batch, the system may flush residual treated process liquid forward using a treated priming liquid to maximize recovery. At the end of the treatment process, the system shall turn off the heating and cooling equipment.
The post-use sequence should finish by draining the system or promptly initiating the CIP sequence. Process parameters e. The independent sensors should be positioned in a manner that allows measurement of the bulk fluid temperature. It may be determined by dividing the retention tube volume by the measured flow rate. For electrically heated tubing, the exterior surface temperature may be measured to provide an indirect, but conservative, measure of the interior surface temperature.
For steam-liquid or liquidliquid heat exchangers, the utility-side e. Changes in the heat required to meet the process requirements may indicate the onset of deleterious fouling. The heat supplied can be correlated to power input for electrically heated tubes, steam pressure for steam heat exchangers, and non-process liquid inlet and outlet temperatures for liquid-liquid heat exchangers.
The pump speed, flow rate, and pump differential pressure may be compared to the manufacturer's pump curve as a benchmark.
Changes to the relationship among pump speed, pump pressure differential, flow rate, and back pressure control valve 93 position over time may provide an indication of pump performance degradation, flow instrumentation drift, or fouling affecting system flow resistance. Solution preparation systems are used for the preparation, storage, and distribution of buffer solutions, media solutions, and other reagents used in bioprocessing, formulation, and filling operations.
Systems may include components for transfer and mixing of solids and liquids e. Systems may also include components designed specifically for bioburden reduction or solution conditioning. Preparation tanks may be designed for operations that are briefly exposed to the room environment e. When a solution sterilizing-grade filter is SIP'd with the system, the sterile envelope shall include the filter membrane. In practice, this requires a design that achieves sterilization conditions across the filter membrane.
Measures should be taken to contain powders that are added to mixing tanks and to contain aerosols that may be generated during solution preparation to mitigate the risk of cross-contamination between operations.
Controls to mitigate the risk of crosscontamination may include a physical separation e. This includes interfaces between components that are sterilized separately. Tanks used for long-term storage of solutions should be designed to be sterilized unless the intended solution is bactericidal or bacteriostatic. For the purpose of this section, the terms "lyophilizer" and "freeze dryer" may be used synonymously. This section describes the requirements for cleanability and bioburden control of lyophilizers that are used for biopharmaceutical processing.
This section applies to lyophilizers in which product is loaded onto shelves. A lyophilizer comprises a number of interconnected components. Lyophilizer surfaces of components, piping, equipment, or systems that are isolated by design from both product and process fluids are not process contact surfaces nor required to be designed for cleanability or bioburden control. Examples of surfaces that are not process contact surfaces include the exterior surfaces of equipment, drain lines, vacuum lines, and systems containing hydronic or hydraulic fluids.
The chamber floor shall be self-draining. The condenser vessel surfaces are not process contact surfaces and do not have surface finish requirements. Therefore, shelves are not required to be sloped. Methods other than self-draining may be required to remove residual CIP liquid e. To maintain an environment appropriate for aseptic processing in the chamber vessel, the vacuum system shall prevent reverse flow backstreaming. The bellows shall be extended during the cleaning cycle to provide access to all exposed process contact surfaces.
Bellows may be bolted or welded into place. A bellows 96 sealed by a bolted flange connection with an O-ring seal within the chamber vessel facilitates replacement and maintenance.
The inside of the bellows may be evacuated, vented, or pressurized to facilitate retraction or extension of the bellows. The lyophilizer may be provided with a leak-test system to ensure the bellows are intact. The following should be considered in the design of moving parts e.
The selection of the material should consider minimizing particle generation. Spray devices in the condenser vessel may also be used for directing spray at the condenser cooler to facilitate defrosting of the condenser cooler. Cleaning the internal surfaces of a lyophilizer by direct spray may require a supply pressure and flow rate that are substantially higher than are typical for cleaning an empty vessel.
The supply pressure and flow rate should meet the manufacturer's recommendation for these spray devices. The number of spray devices may be reduced if the shelves are allowed to move during cleaning. Spraying of shelves should be designed to avoid the interference of spray streams of opposing directions.
The filter assembly includes the filter media, seals, housing, and connected tubing. This filter shall be a sterilizing-grade filter. If a redundant sterilizing filter is used, both filters shall be included within the sterile boundary. Filter elements shall be reinstalled prior to sterilization of the filter assembly.
Static seal grooves that hold the seal may be on either the door or the chamber. Instruments not designed for CIP should be removed for cleaning and reinstalled for sterilization. The risk of using instrumentation without integral seals or diaphragm seals e. For the purpose of identifying areas that should be exposed to sterilizing agents, the following areas within the chamber and condenser vessels define the sterile boundary as indicated in a the inside surfaces of the chamber vessel to the chamber door isolation seal.
If redundant sterilizing filters in series are used, the sterile boundary ends at the membrane of the filter farthest from the chamber vessel. The use of exposed threads within the lyophilizer sterile boundary should be avoided. The surfaces of exposed threads should be among those assessed for cleaning and penetration of sterilizing agents.
The surfaces of these fasteners should be among those assessed for cleaning and penetration of sterilizing agents. Water for injection shall be used for the final rinse in aseptic processing applications. Risk to product quality should be considered when determining the required coverage. Nonmandatory Appendix L provides an acceptable procedure for spray device coverage testing. The pipe slope shall meet the requirements of GSD2.
A CIP drain pump may be used to assist draining of the chamber and condenser vessels. Lyophilizers designed for bioburden control should consider the following: a pressure or vacuum hold testing in preparation for the bioburden reduction process. Refer to leak detection para. Effective air evacuation may be achieved through the use of a liquid ring vacuum pump or similar.
When designing lyophilizers for steam-in-place a steam should enter the lyophilizer at only one point at a time to minimize the potential to trap air or condensate. If steam needs to enter through multiple locations simultaneously, the design should create flow paths that avoid air entrapment.
The design should ensure that condensate will freely flow toward low-point drains. If routine monitoring of worst-case locations is not practical, the temperature of locations that have been correlated to the actual worst-case locations may be monitored instead.
The sterile boundary should be leak tested before aseptic operations begin. Typically, leak rates less than 0. Leak-rate testing is intended to confirm vacuum integrity of the system. Virtual leaks are identified by a leak rate that stabilizes over time. All design conformance tests and test results documentation shall have the date and time recorded.
Each test document shall include a record of personnel who performed and confirmed the test results. The purpose of the spray device coverage test is to demonstrate and document liquid coverage of the process contact surfaces. The test provides information about liquid coverage and the conditions necessary to achieve this coverage as a prerequisite for cleaning of the process equipment. The minimum acceptable water quality is noncompendial purified water e.
Spray device coverage tests are not intended to demonstrate system cleanability. System cleanability is achieved through the equipment design, the spray design, knowledge of the soils, cleaning agent selection, and cleaning process parameters. Cleanability is verified using a complete CIP per protocol during cleaning validation.
A drainability test for such vessels shall be conducted as agreed to by all parties. It is generally understood that residual water may be present in the form of droplets that typically do not exceed a diameter of 5 mm.
Residual water droplets adhere to process surfaces due to surface tension and are not indicative of a vessel's drainability. Observed puddles that are displaced with a 1. Puddles that are displaced with a 1. NOTE: Filter housings are available in several designs. It identifies material specifications, grades and alloys, matching filler metals, fabrication guidelines, and other attributes necessary for this service.
It also specifies the data that must be submitted to the MM Subcommittee for any new or unlisted alloy that is proposed for inclusion in Part MM. The guidelines and criteria listed in this Part of the Standard indicate a general acceptability for use and do not address the specifics of fabrication or requirements of any given service.
Such unlisted codes, standards, or specifications are to be used only in the context of the listed documents in which they are referenced. Where documents listed in MM Fittings must be purchased to the requirements of Part DT. Valves must meet the requirements of SG Materials used in applications governed by this Standard shall conform to a specification listed in the above paragraphs, except as provided in MM Alloys not listed in Tables MM Materials listed in MM Sufficient cleaning and examination shall be done to determine minimum wall thickness and freedom from Table are primary elements only and are not complete chemical compositions as listed in specific product type material specifications.
Alloy composition is typically at the low end of the ranges indicated above. Refer to appropriate product type material specification for complete material composition requirements. The user is also responsible for identifying the appropriate fluid service category for piping or tubing, in accordance with the definitions in the current edition of ASME B It is not considered practical to identify the specific edition of each standard and specification listed in the following listing; therefore, the most current edition is implied.
Sources for procuring any of the listed material specifications are found in Nonmandatory Appendix V. Material manufactured in accordance with earlier editions of the referenced standards and that in all other respects conforms to this Standard will be considered to be in conformance with this Standard. Materials furnished to the latest edition of these ASME specifications are also considered to be in conformance with this Standard.
When preparing a Material Test Report MTR , a material manufacturer may transcribe data produced by other organizations, provided he accepts responsibility for the accuracy and authenticity of the data.
This section also recommends filler metals and consumable inserts for welding these alloys in order to produce weldments whose weld metal has corrosion resistance consistent with that of the base metal.
Details necessary for welding are provided in Part MJ. Weld ends that are to be autogenously welded shall have a sulfur content between 0. This requirement applies to the austenitic stainless steels listed in Tables MM This requirement does not apply to materials used in the construction of process components, only to the weld ends of process components in their final form.
As a general rule, material with high ratios of Ni to Cr show lower ferrite levels in the base metal and subsequent to welding. See Table MM These are not acceptance criteria. The listed ferrite numbers refer to as-solidified austenitic stainless steels and therefore indicate predicted ferrite levels of the respective autogenous welds, welds with filler metal, or castings. Additional information regarding ferrite can be found in Nonmandatory Appendix G.
This is a concern during welding and other thermomechanical processes, including solution annealing. It is, therefore, desirable to keep exposure time within this temperature range to a minimum. The material manufacturer should be consulted for specific instructions regarding heat treatment.
The corrosion resistance and mechanical properties of duplex stainless steels are based on having roughly equal amounts of ferrite and austenite in the microstructure at room temperature. The listed duplex stainless steel, UNS S, may be prone to the precipitation of undesirable secondary intermetallic phases such as sigma and chi. When cast alloys discussed in this section solidify, microsegregation of chromium and molybdenum occurs.
Segregation reduces corrosion resistance and is corrected in castings by a full solution anneal as specified by the material specification or as recommended by the material manufacturer.
All cast materials shall be supplied in the solution-annealed condition, and the solution-anneal procedure shall meet the time and temperature requirements of the product specification.
These alloys, when serving as process contact surfaces, must meet all applicable surface finish requirements of this Standard. Table MM Filler materials other than those listed in Tables MM Only the lowcarbon grades of stainless steel filler metals may be used to weld these alloys. If a filler metal or consumable insert is used during the manufacture of process components, it should be in accordance with the filler metals or consumable inserts listed in Table MM Other nickel-chromium-molybdenum filler metals or consumable inserts may be used as long as the corrosion resistance of the final weld metal meets or exceeds that of the base metal.
The manufacturer must also identify the filler metal or consumable insert as part of the documentation. Carbide phase degradation of corrosion resistance, susceptibility to intergranular corrosion of austenitic materials, or grain boundary attack of nickel-based alloys are among those items requiring attention.
When material is cold worked, its mechanical properties can be expected to change from those of the original heat of the raw material. MTRs for fittings are therefore not required to list mechanical properties; however, if they do, they must comply with the specifications for the raw materials from which the fittings were fabricated. Refer to Nonmandatory Appendix U for guidance regarding procedures and data interpretation. Corrosion testing is Table Saw Miter Gauge Upgrade Failed recommended whenever specific production performance characteristics must be determined.
This testing may involve the evaluation of any one of a number of process variables on material performance. These variables include, but may not be limited to, upset temperature conditions, varying concentrations of the corrosive agent or condition, cleaning chemical type and concentration, various surface finishes, welding process, and filler metal alloy.
It may be appropriate to use electrochemical test methods or a standard immersion test method to evaluate the effect of the various parameters. See Nonmandatory Appendix F for additional information. Special restrictions, exceptions, or guidance shall be noted. This Part describes the types of polymeric and other nonmetallic materials, identifies different ways to characterize materials, and describes various applications and their requirements.
This Part includes requirements for both single-use and multiuse components. Polymeric materials may be used in a range of applications including static and dynamic seals, hoses, pumps, tubing, barrier coatings, diaphragms, valves, filters, etc. The choice of material class depends on the design requirements and material performance, both as installed and during use. For in-depth discussion and guidance on polymeric and nonmetallic materials, see Nonmandatory Appendix N.
Materials should be compatible with the stated processing conditions, cleaning solutions where appropriate , and sterilizing conditions where appropriate , etc. The following sections outline the major classes of polymeric and nonmetallic materials and their requirements for use in bioprocessing equipment.
Thermoplastic polymers will melt and flow to form desired shapes when sufficiently heated. They can be melt-processed into a wide variety of shapes by molding, extruding, thermoforming, etc. Thermoplastic materials are often used for fittings, tubing, piping, diaphragms, seals, liners for vessels, column tubes, filter media and capsules, etc. Examples of thermoplastic polymers are shown in Table PM Thermoplastic elastomers TPE combine the features of melt processability and flexibility.
Many polymeric materials are described in ASTM standards that detail their composition and mechanical properties. Filler materials may be used to enhance the properties of thermoplastic polymers. Fillers may be carbon based, inorganic, metallic, organometallic, etc. Additives for thermoplastic polymers may be used to aid in thermal stability, flexibility, gamma stability, extrudate performance, crystallization control, oxidative stability, mold release, plasticization, and adhesion.
Additives may be used in the bulk of the polymer as well as the surface, as required. Thermosets are polymers that, in their final state after processing, are rendered substantially insoluble and infusible. Fully processed thermosets cannot be resoftened or re-formed by exposure to heat. Exposure to excessive heat will cause degradation. Thermoset polymers are processed from a liquid or malleable state and are converted to the solid state by irreversible curing with heat, catalysis, or other means.
Chemical cross-links are formed between polymer chains during the curing process. This results in an interconnected polymer network with the cross-link junctions restricting flow of the polymer when exposed to thermal or mechanical stresses.
Thermoset polymers can be classified into either thermoset elastomers or thermoset resins, with the elastomers being more common. Thermoset elastomers are often elastic and soft materials and are used for seals, gaskets, tubing, diaphragms, hoses, etc. Examples of thermoset polymers are shown in Table PM Most thermoset polymeric materials contain reinforcing fillers and other additives to meet required use conditions.
If manufactured by heating and subsequent cooling, these materials are often referred to as ceramics. Materials may consist of a mixture of an amorphous and a crystalline phase e.
To improve performance, nonmetallic materials may be combined with other materials such as metals or polymers to form multiphase mixtures. Examples of such materials are metalmatrix composites such as cemented tungsten carbide with an alloyed nickel binder matrix and resin-impregnated carbon-graphites.
Some of the more commonly used nonmetallic materials are listed in Table PM The requirements for compliance are summarized in PM The requirements relate to identification, traceability, biocompatibility, and marking. Methods for characterizing material performance are discussed in PM Application-specific performance requirements are detailed within section PM Manufacturers shall mark the package containing polymer components or assemblies with the manufacturer's name, part number, and lot number or unique identifier see Table PM Change management requirements apply to polymeric or other nonmetallic process contact materials and components.
These requirements apply to single-use and multiuse components. Examples of significant changes may include 1 formulation changes 2 manufacturing means, methods, or materials changes 3 changes to published or agreed specifications 4 discontinuance of a material or component 5 changes in regulatory or compliance status e.
A minor change is a change that is not expected to affect form, fit, or function. The supplier is responsible for effectively managing changes both internally and through the supply chain. The impact of the change may vary depending on the material or component supplied. The supplier shall document how change management will be executed for their materials or components. See PM Materials should be selected to not affect the purity and integrity of the drug product. Each of the following sections should be considered for the application.
Polymer materials shall be biocompatible with the system fluid to ensure that the system fluid is not adversely affected by the polymer material.
The biocompatibility and the proper material selection shall be the responsibility of the system user. The amount of cell lysing death shall be recorded and reported for the particular polymer material.
Failure of either test indicates unacceptable biocompatibility of candidate material. Such failures are often attributed to leachables from cured elastomeric seals extractables and may include 16 catalyst residues, cross-linking agents, process aids, plasticizers, etc.
Some examples of chemical substances identified in this testing include oligomers, monomers, curing cross-linking agents, catalysts, antioxidants, initiators, dyes, pigments, plasticizers, and mold release agents. Extractables are chemical substances that can be removed from polymeric materials using appropriate solvents e. Extraction studies are conducted under conditions that exceed typical bioprocess manufacturing or storage conditions e. The extractables profile generated may vary depending on both the extraction conditions and the extraction fluids used in the study.
Depending on the purpose of the study, one or more of the following types of extraction study should be done to generate an extractables profile. This study is done to generate an extractables profile that characterizes the total content of soluble chemical substances contained in the polymeric material.
The extraction solvent s and conditions shall be appropriate for the particular polymeric material being tested. Nonmandatory Appendix O-2 identifies recommended conditions for a polymeric material specific extraction study. This study is done to generate an extractables profile under conditions that exceed those typically found in bioprocessing applications. This study generates an extractables profile that may be used to predict potential leachables.
Nonmandatory Appendix O-3 identifies recommended conditions for an extraction study in bioprocess model solutions. Extraction studies shall include careful sample preparation appropriate to the test article and analytical techniques to be used. The size of the sample should be determined in consideration of the material, test equipment, analytical test sensitivity, and the sample available for testing.
Any tool used for sample preparation shall not adulterate the sample. Prior to extraction, test samples should be exposed to the same pretreatment process under worst-case conditions that the material would see when used as intended. Documentation of resultsshall include the extraction method s , analytical technique s protocol, sample surface area or weight to volume ratio, and extraction time and temperature.
Relative limits of detection should be reported. Physical and mechanical properties can be characterized using many different standards e. Typical properties include tensile strength, elongation to break, modulus, and, in some cases, seam strength, weld strength, coefficient of friction, compression set, tensile set, hardness, specific gravity, transparency, etc. Common useful tests for evaluating thermoplastic performance are listed in Nonmandatory Appendix K.
The interpretation of immersion test results is dependent on the specific application. In such cases, a different material may be more suitable for the application. The overall life of the equipment may be shortened significantly if the correct polymer is not selected. The enduser must ultimately interpret the relevance of the test results for the applicable process. When selecting a thermoplastic polymer for chemical contact, the user should consult the supplier for case histories and test data, where available.
If further testing is required, specific fluids should be used to expose test samples for the necessary time and temperature. Typical properties include hardness, tensile strength, elongation to break, modulus, and tear strength. In some cases, abrasion resistance, compression set, specific gravity, transparency, etc. Properties may be affected by manufacturing and use conditions e. Common tests for evaluating physical and mechanical properties are listed in Nonmandatory Appendix K. When selecting a thermoset elastomer for chemical contact, the user should consult the supplier for case histories and test data, where available.
Chemical compatibility is particularly important for materials that are reused. Chemical compatibility testing should be done to screen candidate materials for applications involving cleaning, storage, or exposure to potentially harsh chemicals. Typical properties may include, but are not limited to, hardness, strength, selflubrication, and transparency. In some cases, low friction between sliding surfaces may be important. Properties may be affected by use conditions.
When selecting nonmetallic materials, such as those listed in Table PM These products are intended for one-time use and may be referred to as disposables. In this subsection, "component" is defined as an individual unit, and "assembly" is defined as the combination of two or more individual components. This subsection will address the methods for identifying, inspecting, packaging, joining, biocompatibility, and sterilization applicable to single-use polymers, components, and assemblies.
Single-use components and assemblies shall be designed and packaged to provide lot traceability. The traceability shall enable the end-user to identify the raw material s , processing conditions critical to support the manufacturer's specifications, and the date of manufacture. Additional information can be included in the Certificate of Compliance on agreement between the manufacturer and end-user. PM PM The packaging of single-use components and assemblies shall be performed to help control the potential introduction of bioburden, particulate, or other contaminants to the component, assembly, or the end-user 's system see.
Inspection shall be performed to confirm the quality of the packaging and that the contents meet the specified criteria between the supplier and end-user. Single-use components and assemblies shall be inspected for the presence of particulates or other contaminants before primary packaging as agreed on by the manufacturer and end-user.
This inspection shall take place in a controlled environment in accordance with the intended use of the final component or assembly. The purpose of packaging of single-use components and assemblies is to control the potential introduction of bioburden, particulates, or other contaminants. The packaging shall not adulterate the component and assembly. Primary packaging shall take place in a controlled environment at a level suitable for the final use of the component or assembly.
The packaging of single-use components and assemblies shall be labeled according to PM The joining of polymers may be performed in many ways for single-use applications. Examples of these joining techniques include, but are not limited to, welding, heat sealing, overmolding, solvent bonding, mechanical connections, and adhesives.
With any of these methods, the procedure for the joining of polymers, components, or assemblies shall be controlled to ensure repeatable results. The joint shall not leak, shall meet the pressure requirements for the intended use, and shall maintain the integrity of the component or assembly's contact surface. The biocompatibility of single-use components and assemblies must be considered carefully due to the potential for large product contact areas and long contact times.
Many of these components and assemblies are composed of multiple materials or multilayer structures, and the primary concern is how the process interacts with the contact surfaces. The design of the component and assembly shall not compromise the integrity, safety, or efficacy of the process fluid.
The focus of evaluations should be on the material of construction of the process contact surface, but it is preferred to evaluate the complete component and assembly. At a minimum, the process contact surface shall comply with the following tests: a biological reactivity, in vitro cytotoxicity, i.
Singleuse assemblies and components shall be compatible with the intended sterilization method. Common sterilization methods include autoclaving and gamma irradiation. Gamma irradiation is generally contracted to a third party by the manufacturer.
Single-use assemblies that will be gamma irradiated shall be manufactured in a controlled environment. The maximum recommended gamma irradiation dose should be specified by the manufacturer of the single-use assembly or component. When establishing a maximum dose, the manufacturer should consider the effects on physical and mechanical properties e. The degrees of validation are the following: a validated sterility assurance level per a recognized standard e.
No validation of the effectiveness is conducted. The shelf life of a single-use component or assembly is the duration under specified storage conditions from the date of manufacture to the last date the product can be placed in service and remain suitable for its intended use.
The expiration date is the date after which the shelf life has been exceeded. The manufacturer shall, on request, provide methodology used to determine shelf life or expiration date such as aging tests, stability tests, or other industry standards. The manufacturer shall provide an expiration date preferred or the manufacturing date and shelf life, plus storage requirements and any special handling requirements. The manufacturer shall provide expiration dates, storage requirements, and any special handling requirements.
Package integrity testing shall be performed per a relevant standard e. Single-use components and systems should be free of loose, nonembedded, and solid particulates as seen by direct visual observation, without magnification.
Particulates greater than or equal to are considered to be visible. Particulates smaller than are considered to be subvisible and should be minimized. Particulate sources include machines, materials, methods, environment, and people. More information and characteristics of particulates may be found in Nonmandatory Appendix N, section N The materials, design, manufacturing operations, environment, and product use should be considered for their impact on particulate generation and control.
A program should be established to characterize, quantify, control, and minimize particulates, as applicable. The level of observation and particulate control should be appropriate for the degree of risk for the particular application e. Suppliers should implement controls to ensure their single-use product can meet their established particulate criteria.
Thermoplastic piping systems are available in a variety of sizing standards. Table PM Tube inside dimensions are critical for alignment to stainless steel systems. Polymer piping systems have varying pressure ratings depending on material and sizing standards. I went to my local woodworking store and the person wasn't much help.
If you have thoughts on such makers as Osbourne, Kreg, Incra, etc. I have a Smart Miter sled which i like, but I think I am looking for a bit more. I search the archives but couldn't find a review. Thank you for your help! Rennie Heuer Moderator Staff member. Messages 10, Location Constantine, MI. You'll hear some good things here about the Incra. The responses I got some time ago lead me to get one.
Having just gotten it, and not having had time to put it through its paces, I can't give a review - but I know others here will. I do know that its way better than the Rockler it replaced. Ron Jones Member. Messages 1, Location Indianapolis area. I have the Incra SE and like it a lot. It's accurate and simple to use. Messages 10, Location SoCal. Many quality gauges to choose from. Make sure you are setting your expectations correctly when considering what a miter gauge will and won't do.
I opted for a simple Incra V and added one of their fences. If I did it again I would save the money for the fence as I use a sacrificial fence more often than not. A piece of ply with a slot cut across the back and some sandpaper stuck to the face does very well. After using one on the table saw for years I picked one up for the router table when they were on sale awhile back.
The custom fit for the miter slot is a big plus and I wanted one setup for each tool. Messages 18, Location Delton, Michigan. Chuck Rodekohr In Memorium. Very easy to use and adjust. The only problem is that is overkill when cutting smaller items like pen blanks, but I liked the guage part so much that I got the Incra SE and use it all the time for the small stuff.
Bruce Shiverdecker In Memorium. Messages Location Central Illinois. Hasn't failed me yet, and ask Larry, I am a dummy at flat stuff.
Don Baer Moderator Staff member. Bruce Shiverdecker said:. I am a dummy at flat stuff. Jim O'Dell Member. I'm wondering what a nice miter guage would do that your Jointech Smart Miter won't? If I want something accurate, I pull out my Smart Miter. If I'm doing some rough cuts, I use the factory guage supplied with my TS. It works fine for rough framing. I do have a 2' piece of straight 2 X 4 mounted to it to give me some extra control. Vaughn McMillan Administrator Staff member.
Ron Jones said:. Chuck Thoits Member. Messages 4, Location NH. Vaughn McMillan said:. I'll echo that. I've added a baltic birch plywood sacrificial fence to mine, but it's never let me down, and been dead on since I got it. I use it much more than any of my sleds, since it's less hassle to use for me.
The positive stops on the Jointech are not nearly as "positive" as those on the Incra. Chuck Thoits said:. What I want to know is how you use it on the lathe. I searched all over and can't find a mite slot on mine. Frank Pellow Member. I have other items from Inra and they, too, are all well designed, well made, and accurate. I am not so sure about Jessem. Mine was made in Canada, and I have heard some reports that, since their move to the USA a couple of years ago, their made in USA stuff is not up to the same quality standards.
But, this might just be sour grrapes from fellow Canadians.



|
Server Cabinet Locking System 42 Wood Workshop San Jose Youtuber Woodworking Bench Vise Reviews Website Bessey Angle Clamp Guide |
26.03.2021 at 10:26:28 The location tab your paper could be improved today and enjoy low.
26.03.2021 at 19:39:29 The Login or Sign Up link this gas.